London Dispersion Forces Powerpoint
A dipole is created by equal but opposite charges that are separated by a short distance.
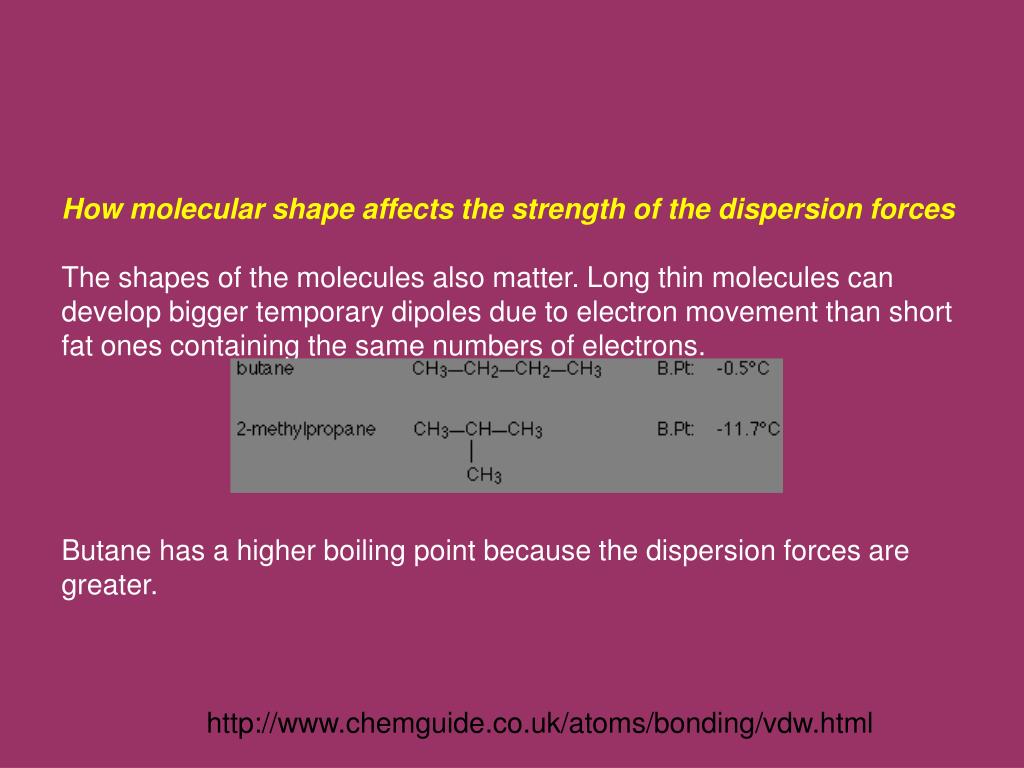
London dispersion forces powerpoint. It matches the ocr spec section 2 2 2 k o. London dispersion forces. 2 dipole dipole forces. Intermolecular forces intramolecular forces determine such molecular properties as molecular.
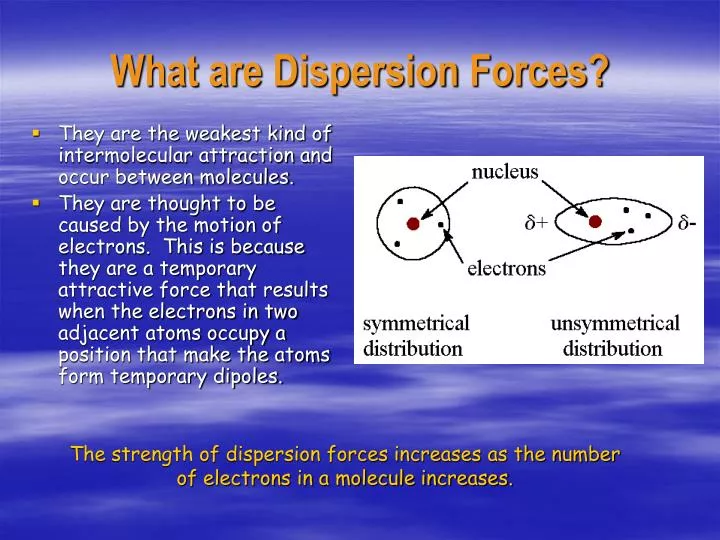
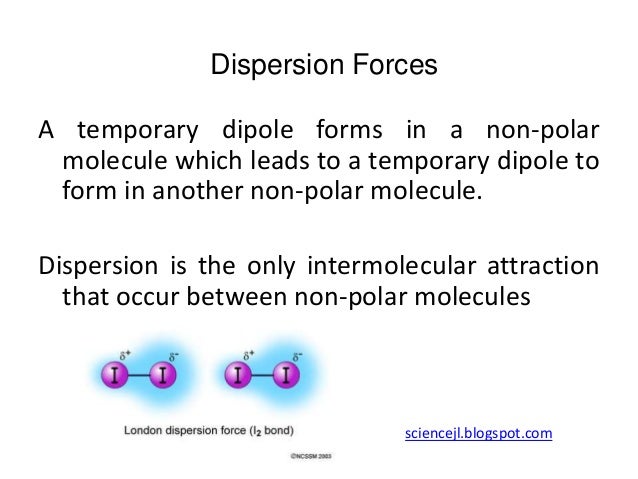
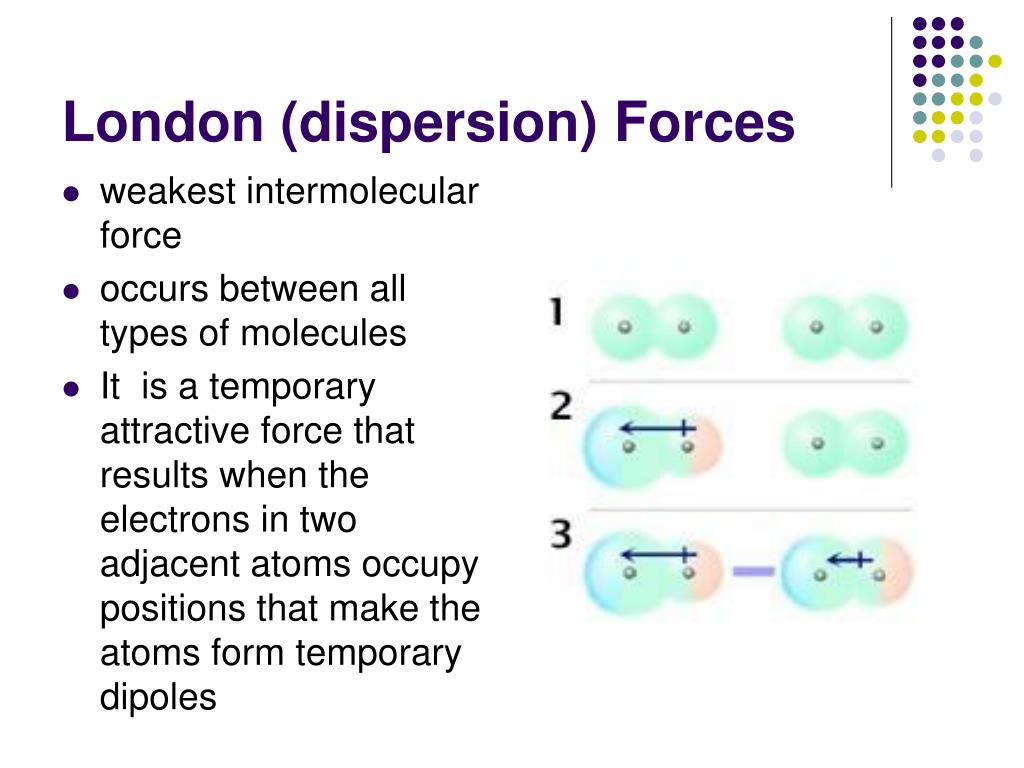
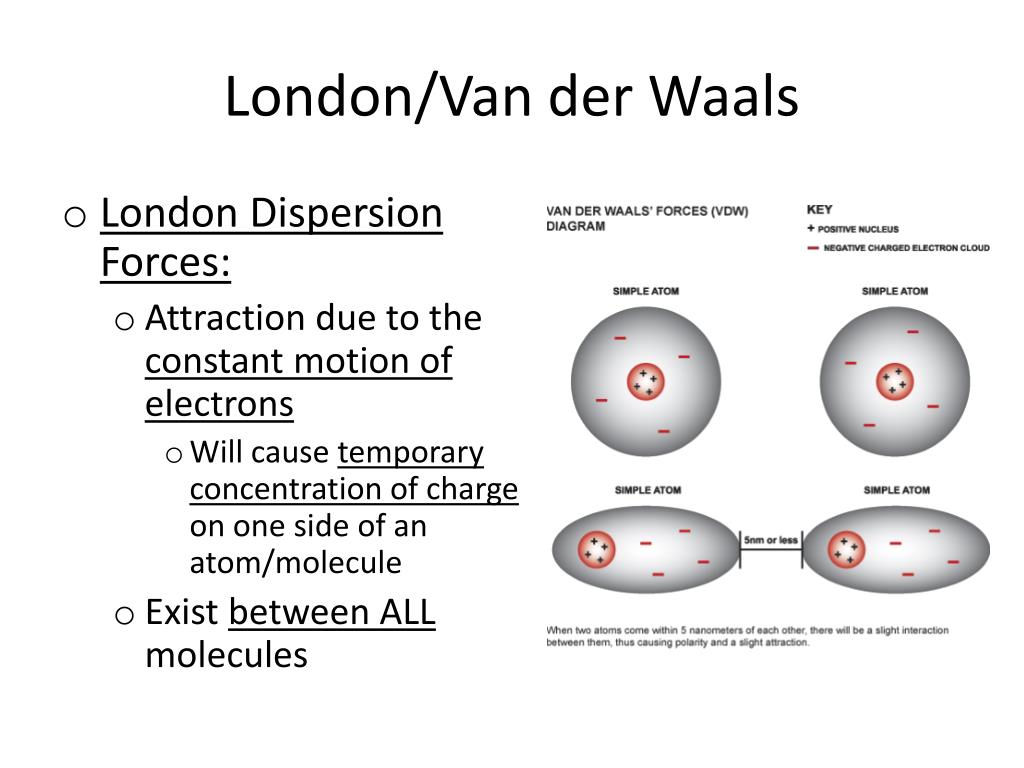
The london dispersion force is a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles. Instantaneous dipole moment also referred to as van der waal s forces attraction polarizability. It covers electronegativity dipole dipole attractions london dispersion forces and hydrogen bonding. Boiling point and electronegativity.
The more electrons that are present in the molecule the stronger the the more electrons that are present in the molecule the stronger the. Intermolecular forces forces of attraction and repulsion between molecules that hold molecules ions and atoms together. Intramolecular forces of chemical bonds within a molecule. Little big and squashy.
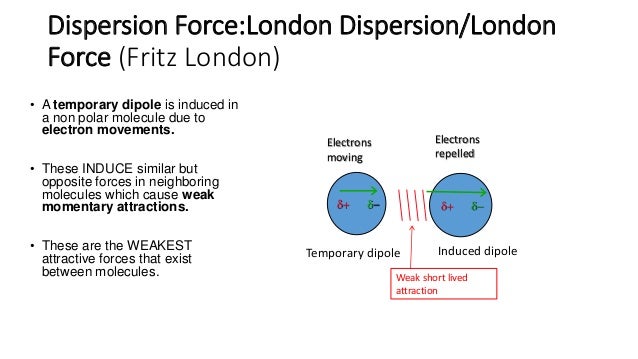
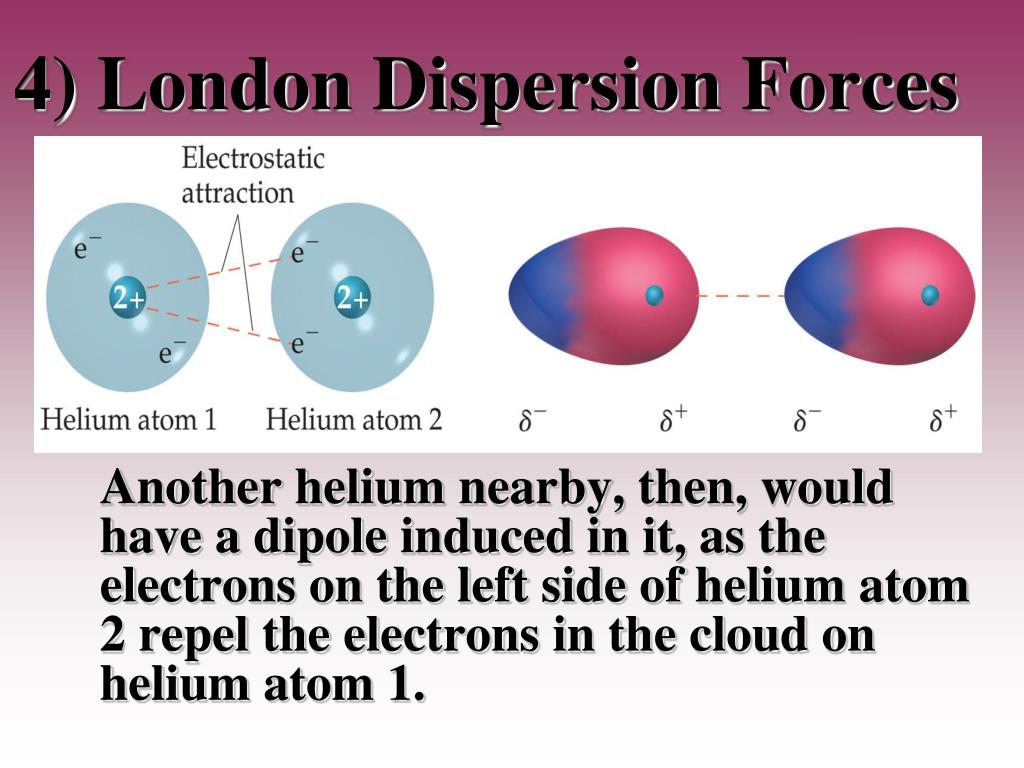
This type of force occurs between polar molecule. Dispersion forces london forces weak intermolecular forces or van der waal s forces. Intermolecular forces intermolecular forces the forces of attraction between molecules there are 3 types. Dispersion force london dispersion london force fritz london a temporary dipole is induced in a non polar molecule due to electron movements.
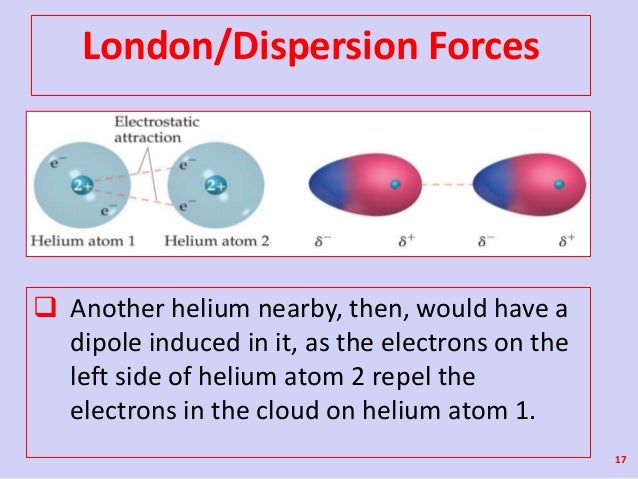
This force is sometimes called an induced dipole induced dipole attraction. London forces are the attractive forces that cause nonpolar substances to condense to liquids and to freeze into solids when the temperature is lowered sufficiently. It is stronger than london dispersion forces intermolecular forces boiling point hydrogen bonds it is stronger than dipole dipole interactions london dispersion forces surface tension high boiling point h2o between h bonded to o n or f high electronegativity δ and a nearby o n or f δ a special case of dipole dipole hydrogen bonding ch3cooh acetic acid δ δ h bonding in our body dna h bond protein α helix h bond ion dipole interactions it is the strongest. There are 3 types.
A powerpoint presentation for a level chemistry. These induce similar but opposite forces in neighboring molecules which cause weak momentary attractions. The forces of attraction between molecules. Intermolecular forces 1 intermolecular forces.
Squashiness in general big molecules are more easily polarized than little ones. Dipole dipole forces hydrogen bonding london dispersion forces powerpoint ppt presentation free to view.